LixiLan-O Pivotal Data
SOLIQUA 100/33 Significantly Reduced A1C Over 30 Weeks1,2

-
74% of patients got to ADA goal (<7%) with SOLIQUA 100/33.1
- 33% of GLP-1 RA (lixisenatide) patients and 59% of Lantus patients got to goal.

Otto, 67
Retired
Not actual patient or profile.
Current treatment
- Metformin 1000 mg BID
- Dapagliflozin 10 mg QD
- Lisinopril 20 mg QD
- Atorvastatin 10 mg QD
- A1C: 9.2%
- FPG: 154 mg/dL
- PPG: 262 mg/dL
- BMI: 31 kg/m2
- Duration of diabetes: 5 to 7 years
- High blood glucose levels at 1 to 2 points during the day (~2 hours after meal); levels also slightly elevated at night (~9 PM)
- When considering new medications, has concerns about co-pays/out-of-pocket costs
- Medicare Advantage Prescription Drug Plan
Physical & lab evaluation
Patient History
Insurance
LixiLan-O Pivotal Study
-
LixiLan-O Post Hoc Subgroup Analysis
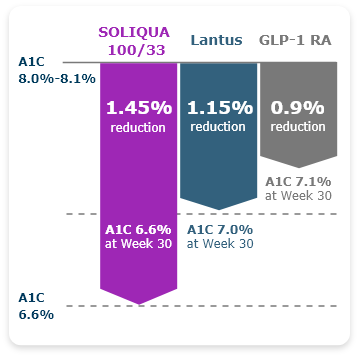
Greater A1C Reductions Were Observed With SOLIQUA 100/33 in Older Adults (≥65) With T2DM vs Lantus or a GLP-1 RA3

LixiLan-O Post Hoc Subgroup Analysis
Greater A1C Reductions Were Observed With SOLIQUA 100/33 in Older Adults (≥65) With T2DM vs Lantus or a GLP-1 RA3

-
LixiLan-O Post Hoc Subgroup Analysis
SOLIQUA 100/33 Was Up to 2x More Likely to Help Older Adults (≥65) With T2DM Reach Goal Than Lantus or a GLP-1 RA Alone3

~1.4x and ~2x more patients reached goal with SOLIQUA 100/33 vs Lantus and GLP-1 RA, respectively.1
LixiLan-O Post Hoc Subgroup Analysis
SOLIQUA 100/33 Was Up to 2x More Likely to Help Older Adults (≥65) With T2DM Reach Goal Than Lantus or a GLP-1 RA Alone3

~1.4x and ~2x more patients reached goal with SOLIQUA 100/33 vs Lantus and GLP-1 RA, respectively.1
-
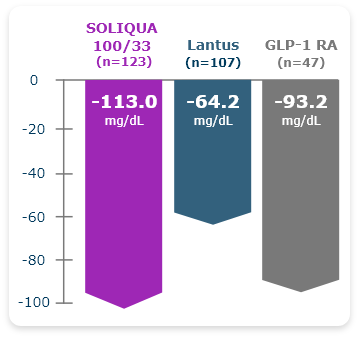
LixiLan-O Post Hoc Subgroup Analysis
SOLIQUA 100/33 More Effectively Lowered PPG Levels vs Lantus or a GLP-1 RA in Older Adults With T2DM3

~1.2x and ~2x improvement with SOLIQUA vs GLP-1 RA and Lantus, respectively.1
LixiLan-O Post Hoc Subgroup Analysis
SOLIQUA 100/33 More Effectively Lowered PPG Levels vs Lantus or a GLP-1 RA in Older Adults With T2DM3

~1.2x and ~2x improvement with SOLIQUA vs GLP-1 RA and Lantus, respectively.1
-
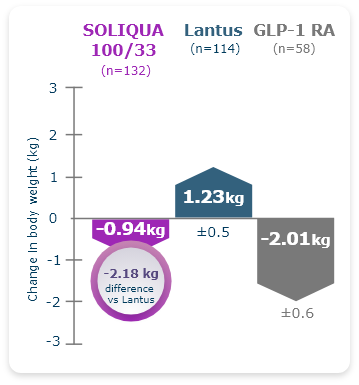
LixiLan-O Post Hoc Subgroup Analysis
Reduction in A1C Without Additional Weight Gain Was Observed in Older Adults (≥65) With T2DM Taking SOLIQUA 100/333

LixiLan-O Post Hoc Subgroup Analysis
Reduction in A1C Without Additional Weight Gain Was Observed in Older Adults (≥65) With T2DM Taking SOLIQUA 100/333

-
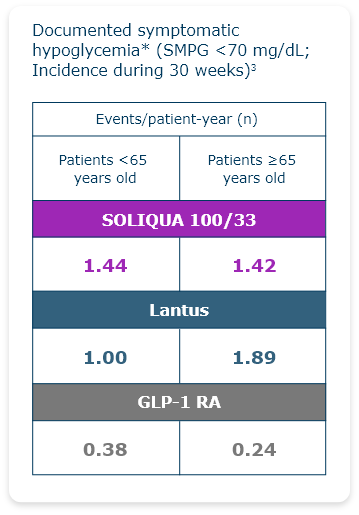
LixiLan-O Post Hoc Subgroup Analysis
Reduced Hypoglycemic Events Were Observed in Older Adults (≥65) With T2DM3

Hypoglycemia is the most common adverse event with insulin-containing therapy.1
- Documented symptomatic hypoglycemia was defined as typical symptoms of hypoglycemia accompanied by an SMPG value of <70 mg/dL.
LixiLan-O Post Hoc Subgroup Analysis
Reduced Hypoglycemic Events Were Observed in Older Adults (≥65) With T2DM3

Hypoglycemia is the most common adverse event with insulin-containing therapy.1
- Documented symptomatic hypoglycemia was defined as typical symptoms of hypoglycemia accompanied by an SMPG value of <70 mg/dL.

Omar, 58
Teacher and coach
Not actual patient or profile.
Current treatment
- Metformin 1000 mg BID
- Rosuvastatin 10 mg QD
- Dapagliflozin 10 mg QD
- Olmesartan 40 mg QD
- A1C: 9.4%
- FPG: 152 mg/dL
- PPG: 245 mg/dL
- BMI: 29 kg/m2
- Duration of diabetes: 8 years
- A1C continues to rise and has surpassed 9% despite treatment with OADs
- Patient reports having less energy and feeling sluggish
- Doctor prescribed using a CGM for 14 days and identified excursions in Omar’s FPG and PPG levels
- Determined to reach A1C goal because of concerns about fluctuations in his blood glucose levels
- PPO
Physical & lab evaluation
Patient History
Insurance
-
LixiLan-O Post Hoc Subgroup Analysis
A 2.9% A1C Reduction Was Observed With SOLIQUA 100/33 in Adults With A1C ≥9%4

LixiLan-O Post Hoc Subgroup Analysis
A 2.9% A1C Reduction Was Observed With SOLIQUA 100/33 in Adults With A1C ≥9%4

-
LixiLan-O Post Hoc Subgroup Analysis
More Patients Who Have A1C ≥9% Achieved ADA Goal (7% A1C) With SOLIQUA 100/334

LixiLan-O Post Hoc Subgroup Analysis
More Patients Who Have A1C ≥9% Achieved ADA Goal (7% A1C) With SOLIQUA 100/334

-
LixiLan-O Post Hoc Subgroup Analysis
Patients with A1C ≥9% Taking SOLIQUA 100/33 Experienced Improved Glycemic Values Throughout the Day5

LixiLan-O Post Hoc Subgroup Analysis
Patients with A1C ≥9% Taking SOLIQUA 100/33 Experienced Improved Glycemic Values Throughout the Day5

- Eligible patients (N=1479) were enrolled in a 4-week run-in period, during which they stopped taking any second OAD, and titrated metformin to ≥2000 mg/dL or maximum tolerated dose (≥1500 mg/dL).2
- Patients inadequately controlled at the end of the run-in period (N=1170) were randomized to either SOLIQUA 100/33 (n=469), Lantus (n=467), or lixisenatide (n=234) with metformin.2
- Criteria for randomization2:
- A1C between 7% and 10%.
- FPG ≤250 mg/dL.
- The maximum allowable insulin glargine dose was 60 Units for both the SOLIQUA 100/33 and Lantus treatment groups.1

Post Hoc Analysis Description: In this post hoc subgroup analysis of LixiLan-O patients ≥65, efficacy endpoints were changes from baseline in A1C, FPG, and PPG; the proportion of patients achieving A1C goal; and change in body weight. Safety measures assessed were the incidence of documented symptomatic hypoglycemia or severe hypoglycemia and the incidence of gastrointestinal adverse events.3
Post Hoc Analysis Limitations: This study was not designed or powered to detect differences between treatments within this subgroup. The difference in effect observed in this subgroup analysis may not necessarily be reflected in the care setting where alternative insulin glargine dosage can be used.3
When A1C Is High, the ADA Recommends the Use of a Basal Insulin Plus a GLP-1 RA to Get A1C Down6
The 2023 ADA Guidelines recommend initial injectable combinations, such as basal insulin plus a GLP-1 RA, when A1C is ≥1.5% above target.
SOLIQUA 100/33 is a combination of insulin glargine, an insulin analog, and lixisenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use:
- Concomitant use in combination with any other product containing a GLP-1 receptor agonist is not recommended.
- Not recommended for the treatment of diabetic ketoacidosis.
- Has not been studied in combination with prandial insulin.
Important Safety Information
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
Contraindications
- During episodes of hypoglycemia.
- In patients with known serious hypersensitivity to insulin glargine, lixisenatide, or to any of the product components.
Warnings and Precautions
- Anaphylaxis and Serious Hypersensitivity Reactions: In clinical trials of lixisenatide, there have been cases of anaphylaxis and other serious hypersensitivity reactions including angioedema. Severe, life threatening, generalized allergic reactions, including anaphylaxis and angioedema, can occur with insulins, including insulin glargine. There have been reports of serious hypersensitivity reactions, including anaphylactic reactions and angioedema, in patients treated with SOLIQUA 100/33. If hypersensitivity reactions occur, discontinue SOLIQUA 100/33. Use caution in patients with a history of anaphylaxis or angioedema with another glucagon-like peptide-1 (GLP-1) receptor agonist (RA) because it is unknown whether such patients will be predisposed to anaphylaxis.
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 RAs. After initiation of SOLIQUA 100/33, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, discontinue SOLIQUA 100/33 and initiate appropriate management.
- Never Share a SOLIQUA 100/33 SoloStar® Pen between Patients: Pen-sharing poses a risk for transmission of blood-borne pathogens, even if the needle is changed.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Changes should be made cautiously, and the frequency of blood glucose monitoring should be increased. Adjustments in concomitant oral antidiabetic treatment may be needed. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
- Medication Errors: SOLIQUA 100/33 contains two drugs. Do not administer more than 60 units of SOLIQUA 100/33, which may result in overdose of the lixisenatide component. Do not use other GLP-1 RAs. Accidental mix-ups between insulin products have been reported. Instruct patients to always check the label before administration. Do not withdraw SOLIQUA 100/33 from the pen with a syringe.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction associated with insulin-containing therapy, which may be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity, and in patients with renal or hepatic impairment and hypoglycemia unawareness.
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with GLP-1 receptor agonists. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting or diarrhea. Monitor renal function in patients reporting adverse reactions to SOLIQUA 100/33 that could lead to volume depletion, especially during dosage initiation and escalation of SOLIQUA 100/33. SOLIQUA 100/33 is not recommended in patients with end-stage renal disease.
- Severe Gastrointestinal Adverse Reactions: Use of GLP-1 receptor agonists, including SOLIQUA 100/33, has been associated with gastrointestinal adverse reactions, sometimes severe. SOLIQUA 100/33 is not recommended in patients with severe gastroparesis.
- Immunogenicity: Patients may develop antibodies to insulin and lixisenatide. If there is worsening glycemic control or failure to achieve targeted glycemic control, significant injection site reactions or allergic reactions, then other antidiabetic therapy should be considered.
- Hypokalemia: All insulin containing products can cause hypokalemia, which may be life-threatening. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) and insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and post-marketing. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: SOLIQUA 100/33 delays gastric emptying. There have been rare post marketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking SOLIQUA 100/33.
Most Common Adverse Reactions
The most common adverse reactions reported in ≥ 5% of patients treated with SOLIQUA 100/33 include hypoglycemia, nausea, nasopharyngitis, diarrhea, upper respiratory tract infection, and headache.
Drug Interactions
- Certain drugs may affect glucose metabolism, requiring dose adjustment of SOLIQUA 100/33 and close monitoring of blood glucose.
- The signs of hypoglycemia may be reduced or absent in patients taking anti-adrenergic drugs (eg, betablockers, clonidine, guanethidine, and reserpine).
- The lixisenatide in SOLIQUA 100/33 delays gastric emptying, which may reduce the rate of absorption of orally administered medication with a narrow therapeutic ratio or that require careful clinical monitoring. If such medications are to be administered with food, do not co-administer with SOLIQUA 100/33.
- Antibiotics, acetaminophen, or other medications that are dependent on threshold concentrations for efficacy, or where a delay in effect is undesirable, should be administered at least 1 hour before SOLIQUA 100/33 injection.
- Oral contraceptives should be taken at least 1 hour before SOLIQUA 100/33 administration or 11 hours after.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
Abbreviations: ADA, American Diabetes Association; A1C, glycated hemoglobin; BID, twice per day; BMI, body mass index; CGM, continuous glucose monitor; FPG, fasting plasma glucose; GLP-1, glucagon-like peptide-1; OAD, oral antidiabetic drug; PPG, post-prandial glucose; PPO, preferred provider organization; QD, daily; RA, receptor agonist; SMPG, self-monitored plasma glucose; T2DM, type 2 diabetes mellitus.
References:
- SOLIQUA 100/33 Prescribing Information.
- Data on File CSR 12404. Sanofi US.
- Handelsman Y, Chovanes C, Dex T, et al. Efficacy and safety of insulin glargine/lixisenatide (iGlarLixi) fixed-ratio combination in older adults with type 2 diabetes. J Diabetes Complications. 2019;33(3):236-242.
- Davies MJ, Russell-Jones D, Barber TM, et al. Glycaemic benefit of iGlarLixi in insulin-naive type 2 diabetes patients with high HbA1c or those with inadequate glycaemic control on two oral antihyperglycaemic drugs in the LixiLan-O randomized trial. Diabetes Obes Metab. 2019;21:1967-1972. doi:10.1111/dom.13791
- Aronson R, Umpierrez G, Stager W, Kovatchev B. Insulin glargine/lixisenatide fixed-ratio combination improves glycaemic variability and control without increasing hypoglycaemia. Diabetes Obes Metab. 2019;21:726-731. doi:10.1111/dom.13580
- American Diabetes Association. Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl1):S1-S292.
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
Contraindications
- During episodes of hypoglycemia.
- In patients with known serious hypersensitivity to insulin glargine, lixisenatide, or to any of the product components.
Warnings and Precautions
- Anaphylaxis and Serious Hypersensitivity Reactions: In clinical trials of lixisenatide, there have been cases of anaphylaxis and other serious hypersensitivity reactions including angioedema. Severe, life threatening, generalized allergic reactions, including anaphylaxis and angioedema, can occur with insulins, including insulin glargine. There have been reports of serious hypersensitivity reactions, including anaphylactic reactions and angioedema, in patients treated with SOLIQUA 100/33. If hypersensitivity reactions occur, discontinue SOLIQUA 100/33. Use caution in patients with a history of anaphylaxis or angioedema with another glucagon-like peptide-1 (GLP-1) receptor agonist (RA) because it is unknown whether such patients will be predisposed to anaphylaxis.
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 RAs. After initiation of SOLIQUA 100/33, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, discontinue SOLIQUA 100/33 and initiate appropriate management.
- Never Share a SOLIQUA 100/33 SoloStar® Pen between Patients: Pen-sharing poses a risk for transmission of blood-borne pathogens, even if the needle is changed.
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Changes should be made cautiously, and the frequency of blood glucose monitoring should be increased. Adjustments in concomitant oral antidiabetic treatment may be needed. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
- Medication Errors: SOLIQUA 100/33 contains two drugs. Do not administer more than 60 units of SOLIQUA 100/33, which may result in overdose of the lixisenatide component. Do not use other GLP-1 RAs. Accidental mix-ups between insulin products have been reported. Instruct patients to always check the label before administration. Do not withdraw SOLIQUA 100/33 from the pen with a syringe.
- Hypoglycemia: Hypoglycemia is the most common adverse reaction associated with insulin-containing therapy, which may be life-threatening. Increase frequency of glucose monitoring with changes to: insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity, and in patients with renal or hepatic impairment and hypoglycemia unawareness.
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with GLP-1 receptor agonists. The majority of the reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting or diarrhea. Monitor renal function in patients reporting adverse reactions to SOLIQUA 100/33 that could lead to volume depletion, especially during dosage initiation and escalation of SOLIQUA 100/33. SOLIQUA 100/33 is not recommended in patients with end-stage renal disease.
- Severe Gastrointestinal Adverse Reactions: Use of GLP-1 receptor agonists, including SOLIQUA 100/33, has been associated with gastrointestinal adverse reactions, sometimes severe. SOLIQUA 100/33 is not recommended in patients with severe gastroparesis.
- Immunogenicity: Patients may develop antibodies to insulin and lixisenatide. If there is worsening glycemic control or failure to achieve targeted glycemic control, significant injection site reactions or allergic reactions, then other antidiabetic therapy should be considered.
- Hypokalemia: All insulin containing products can cause hypokalemia, which may be life-threatening. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
- Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) and insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and post-marketing. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: SOLIQUA 100/33 delays gastric emptying. There have been rare post marketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking SOLIQUA 100/33.
Most Common Adverse Reactions
The most common adverse reactions reported in ≥ 5% of patients treated with SOLIQUA 100/33 include hypoglycemia, nausea, nasopharyngitis, diarrhea, upper respiratory tract infection, and headache.
Drug Interactions
- Certain drugs may affect glucose metabolism, requiring dose adjustment of SOLIQUA 100/33 and close monitoring of blood glucose.
- The signs of hypoglycemia may be reduced or absent in patients taking anti-adrenergic drugs (eg, betablockers, clonidine, guanethidine, and reserpine).
- The lixisenatide in SOLIQUA 100/33 delays gastric emptying, which may reduce the rate of absorption of orally administered medication with a narrow therapeutic ratio or that require careful clinical monitoring. If such medications are to be administered with food, do not co-administer with SOLIQUA 100/33.
- Antibiotics, acetaminophen, or other medications that are dependent on threshold concentrations for efficacy, or where a delay in effect is undesirable, should be administered at least 1 hour before SOLIQUA 100/33 injection.
- Oral contraceptives should be taken at least 1 hour before SOLIQUA 100/33 administration or 11 hours after.
Click here for full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
Abbreviations: ADA, American Diabetes Association; A1C, glycated hemoglobin; BID, twice per day; BMI, body mass index; CGM, continuous glucose monitor; FPG, fasting plasma glucose; GLP-1, glucagon-like peptide-1; OAD, oral antidiabetic drug; PPG, post-prandial glucose; PPO, preferred provider organization; QD, daily; RA, receptor agonist; SMPG, self-monitored plasma glucose; T2DM, type 2 diabetes mellitus.
References:
- SOLIQUA 100/33 Prescribing Information.
- Data on File CSR 12404. Sanofi US.
- Handelsman Y, Chovanes C, Dex T, et al. Efficacy and safety of insulin glargine/lixisenatide (iGlarLixi) fixed-ratio combination in older adults with type 2 diabetes. J Diabetes Complications. 2019;33(3):236-242.
- Davies MJ, Russell-Jones D, Barber TM, et al. Glycaemic benefit of iGlarLixi in insulin-naive type 2 diabetes patients with high HbA1c or those with inadequate glycaemic control on two oral antihyperglycaemic drugs in the LixiLan-O randomized trial. Diabetes Obes Metab. 2019;21:1967-1972. doi:10.1111/dom.13791
- Aronson R, Umpierrez G, Stager W, Kovatchev B. Insulin glargine/lixisenatide fixed-ratio combination improves glycaemic variability and control without increasing hypoglycaemia. Diabetes Obes Metab. 2019;21:726-731. doi:10.1111/dom.13580
- American Diabetes Association. Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl1):S1-S292.