How do I take SOLIQUA 100/33?
It is important to inject your dose of SOLIQUA 100/33 under the skin (subcutaneously) once a day within 1 hour before your first meal of the day.
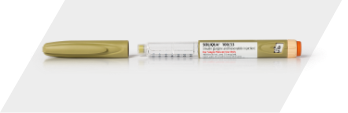
The SOLIQUA 100/33 SoloStar pen
For a daily dose range of 15 to 60 Units. Each pen contains 300 Units of medicine.

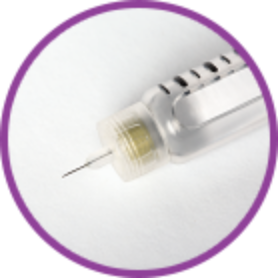
Small thin needle* that goes just under the skin.
*Needle not included with pen. Always use needles compatible with your pen.
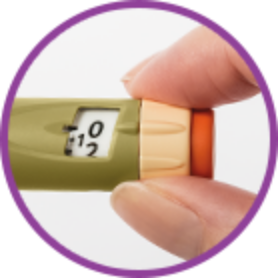
Dose selector dial and push-button injection
work together so you can select and inject your prescribed dose.

Large dose window
lets you clearly see your selected dose. Each line in the dose window equals 1 unit of SOLIQUA 100/33.
Have questions about SOLIQUA 100/33?
It’s natural to have questions when learning how to inject a new medicine for the first time.
Watch our step-by-step video to help you get started.

Getting started on SOLIQUA 100/33?
Download a suite of materials to help manage your T2DM and feel supported.
- Do not dial your dose by counting the clicks or you may dial the wrong dose.
- Always check the number in the dose window to make sure you dial the correct dose.
- As you dial your dose, you may notice that the dose window is black between 2 Units and 15 Units. This is because SOLIQUA 100/33 should not be used for a dose of less than 15 Units.
- Read all the information that came with your pen, including the Instructions for Use.
- Speak with your doctor or healthcare team if you have questions about your pen or do not understand the instructions.
- Know your dose and don’t change your dose unless directed by your doctor.
- Do not reuse needles.
- Do not use a syringe to remove medicine from your pen.
- Do not share your pen with other people, even if the needle has been changed.
- Check the label on the SOLIQUA 100/33 pen each time you inject to make sure you are using the correct medicine.
- If you have problems handling the pen or can’t see the numbers in the dose window clearly, have another person prepare and inject your dose.
- Finding the right dose can take some time. Stay in close contact with your doctor about how you feel so they can adjust your dose to find the dose that works best for you.
- Store with cap in original packaging.
- Refrigerate between 36°F and 46°F (2°C and 8°C).
- Do not freeze. If you accidentally freeze your pen, throw it away.
- Discard if past the expiration date and use a new pen.
- Do not put the pen back in the refrigerator.
- Keep your pen at room temperature below 77°F (25°C).
- Do not store your pen with the needle attached.
- Store with cap on to protect from light.
- Discard the pen 28 days after first use.
Before you use SOLIQUA 100/33
Your healthcare team should teach you how to inject SOLIQUA 100/33. However, before you use your pen:
Take special note:

Before you use your pen for the first time:
After you use your pen:
Always keep your pen and all medicines out of the sight and reach of children.
SOLIQUA 100/33 is an injectable prescription medicine that contains 2 diabetes medicines, insulin glargine and lixisenatide, which may improve blood sugar (glucose) control in adults with type 2 diabetes when used with diet and exercise.
- It has not been studied in people with a history of pancreatitis.
- It is not recommended for people who also take lixisenatide or other medicines called GLP-1 receptor agonists.
- It is not for use in people with type 1 diabetes, or people with diabetic ketoacidosis.
- It has not been studied in people who have a stomach problem that causes slow emptying (gastroparesis) and is not for people with slow emptying of the stomach.
- It has not been studied in people who also take a short-acting (prandial) insulin.
- It is not known if SOLIQUA 100/33 is safe and effective in children under 18 years of age.
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
What is the most important information I should know about SOLIQUA 100/33?
Do not share your SOLIQUA 100/33 pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
SOLIQUA 100/33 can cause serious side effects, including inflammation of the pancreas, which may be severe and lead to death.
Before using SOLIQUA 100/33, tell your doctor if you have had pancreatitis, stones in your gallbladder (cholelithiasis), or a history of alcoholism. These medical problems may make you more likely to get pancreatitis.
Stop taking SOLIQUA 100/33 and call your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe, and will not go away. The pain may be felt in the back area. The pain may happen with or without vomiting.
Who should not use SOLIQUA 100/33?
Do not use SOLIQUA 100/33 if you:
- are having an episode of low blood sugar (hypoglycemia)
- are allergic to insulin glargine, lixisenatide, or any of the ingredients in SOLIQUA 100/33. Symptoms of a severe allergic reaction with SOLIQUA 100/33 may include swelling of the face, lips, tongue, or throat, fainting or feeling dizzy, problems breathing or swallowing, very rapid heartbeat, severe rash or itching, or low blood pressure.
Before using SOLIQUA 100/33, tell your healthcare provider about all your medical conditions, including if you:
- have or have had problems with your pancreas, your kidneys, or your liver, stones in your gallbladder, or a history of alcoholism.
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take thiazolidinediones (TZDs).
- have severe problems with your stomach, such as slowed emptying of your stomach or problems digesting food.
- are taking certain medicines called glucagon-like peptide 1 receptor agonists (GLP-1 receptor agonists).
- have had an allergic reaction to a GLP-1 receptor agonist.
- are pregnant or breastfeeding or plan to become pregnant or to breastfeed. It is not known if SOLIQUA 100/33 will harm your unborn baby or pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including all prescription and over-the-counter medicines, vitamins, and herbal supplements. SOLIQUA 100/33 may affect the way some medicines work. Before using SOLIQUA 100/33, talk to your healthcare provider about low blood sugar and how to manage it.
How should I use SOLIQUA 100/33?
- Do not change your dose without first talking to your healthcare provider.
- Check the pen label each time you inject to make sure you are using the correct medicine.
- Do not take more than 60 units of SOLIQUA 100/33 each day. Do not take SOLIQUA 100/33 with other GLP-1 receptor agonists.
- Only use SOLIQUA 100/33 that is clear and colorless to almost colorless. If you see small particles, return it to your pharmacy for replacement.
- Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- Do not remove SOLIQUA 100/33 from the pen with a syringe.
- Do not re-use or share needles with other people. You may give other people a serious infection, or get a serious infection from them.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check.
What are the possible side effects of SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects including:
- See "What is the most important information I should know about SOLIQUA 100/33?"
- Severe allergic reactions. Severe allergic reactions can happen with SOLIQUA 100/33. Stop taking SOLIQUA 100/33 and get medical help right away if you have any symptoms of a severe allergic reaction. See "Who should not use SOLIQUA 100/33?"
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar is higher if you take another medicine that can cause low blood sugar. Signs and symptoms of low blood sugar include:
- headache
- dizzines
- drowsiness
- sweating
- weakness
- irritability
- hunger
- blurred vision
- fast heartbeat
- feeling jittery
- confusion
- anxiety
- headache
- drowsiness
- weakness
- hunger
- fast heartbeat
- confusion
- dizziness
- sweating
- irritability
- blurred vision
- feeling jittery
- anxiety
- Kidney problems (kidney failure). In people who have kidney problems, the occurrence of diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse.
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called TZDs with SOLIQUA 100/33 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with SOLIQUA 100/33. Your healthcare provider should monitor you closely while you are taking TZDs with SOLIQUA 100/33. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, or sudden weight gain. Treatment with TZDs and SOLIQUA 100/33 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
- Gallbladder problems. Gallbladder problems have happened in some people who take SOLIQUA 100/33. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- yellowing of skin or eyes (jaundice)
- fever
- clay-colored stools
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
The most common side effects of SOLIQUA 100/33 include:
|
|
|
|
|
|
|
|
|
|
|
|
Nausea and diarrhea usually happen more often when you first start using SOLIQUA 100/33.
These are not all the possible side effects of SOLIQUA 100/33. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 1-800-FDA-1088.
Click here for full Prescribing Information for SOLIQUA 100/33.
Click here for information on Sharps Medical WSharps_and_Medical_Wasteaste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
What is the most important information I should know about SOLIQUA 100/33?
Do not share your SOLIQUA 100/33 pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
SOLIQUA 100/33 can cause serious side effects, including inflammation of the pancreas, which may be severe and lead to death.
Before using SOLIQUA 100/33, tell your doctor if you have had pancreatitis, stones in your gallbladder (cholelithiasis), or a history of alcoholism. These medical problems may make you more likely to get pancreatitis.
Stop taking SOLIQUA 100/33 and call your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe, and will not go away. The pain may be felt in the back area. The pain may happen with or without vomiting.
Who should not use SOLIQUA 100/33?
Do not use SOLIQUA 100/33 if you:
- are having an episode of low blood sugar (hypoglycemia)
- are allergic to insulin glargine, lixisenatide, or any of the ingredients in SOLIQUA 100/33. Symptoms of a severe allergic reaction with SOLIQUA 100/33 may include swelling of the face, lips, tongue, or throat, fainting or feeling dizzy, problems breathing or swallowing, very rapid heartbeat, severe rash or itching, or low blood pressure.
Before using SOLIQUA 100/33, tell your healthcare provider about all your medical conditions, including if you:
- have or have had problems with your pancreas, your kidneys, or your liver, stones in your gallbladder, or a history of alcoholism.
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take thiazolidinediones (TZDs).
- have severe problems with your stomach, such as slowed emptying of your stomach or problems digesting food.
- are taking certain medicines called glucagon-like peptide 1 receptor agonists (GLP-1 receptor agonists).
- have had an allergic reaction to a GLP-1 receptor agonist.
- are pregnant or breastfeeding or plan to become pregnant or to breastfeed. It is not known if SOLIQUA 100/33 will harm your unborn baby or pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including all prescription and over-the-counter medicines, vitamins, and herbal supplements. SOLIQUA 100/33 may affect the way some medicines work. Before using SOLIQUA 100/33, talk to your healthcare provider about low blood sugar and how to manage it.
How should I use SOLIQUA 100/33?
- Do not change your dose without first talking to your healthcare provider.
- Check the pen label each time you inject to make sure you are using the correct medicine.
- Do not take more than 60 units of SOLIQUA 100/33 each day. Do not take SOLIQUA 100/33 with other GLP-1 receptor agonists.
- Only use SOLIQUA 100/33 that is clear and colorless to almost colorless. If you see small particles, return it to your pharmacy for replacement.
- Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- Do not remove SOLIQUA 100/33 from the pen with a syringe.
- Do not re-use or share needles with other people. You may give other people a serious infection, or get a serious infection from them.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check.
What are the possible side effects of SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects including:
- See "What is the most important information I should know about SOLIQUA 100/33?"
- Severe allergic reactions. Severe allergic reactions can happen with SOLIQUA 100/33. Stop taking SOLIQUA 100/33 and get medical help right away if you have any symptoms of a severe allergic reaction. See "Who should not use SOLIQUA 100/33?"
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar is higher if you take another medicine that can cause low blood sugar. Signs and symptoms of low blood sugar include:
- headache
- dizzines
- drowsiness
- sweating
- weakness
- irritability
- hunger
- blurred vision
- fast heartbeat
- feeling jittery
- confusion
- anxiety
- headache
- drowsiness
- weakness
- hunger
- fast heartbeat
- confusion
- dizziness
- sweating
- irritability
- blurred vision
- feeling jittery
- anxiety
- Kidney problems (kidney failure). In people who have kidney problems, the occurrence of diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse.
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called TZDs with SOLIQUA 100/33 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with SOLIQUA 100/33. Your healthcare provider should monitor you closely while you are taking TZDs with SOLIQUA 100/33. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, or sudden weight gain. Treatment with TZDs and SOLIQUA 100/33 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
- Gallbladder problems. Gallbladder problems have happened in some people who take SOLIQUA 100/33. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- yellowing of skin or eyes (jaundice)
- fever
- clay-colored stools
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
The most common side effects of SOLIQUA 100/33 include:
|
|
|
|
|
|
|
|
|
|
|
|
Nausea and diarrhea usually happen more often when you first start using SOLIQUA 100/33.
These are not all the possible side effects of SOLIQUA 100/33. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 1-800-FDA-1088.
Click here for full Prescribing Information for SOLIQUA 100/33.
Click here for information on Sharps Medical WSharps_and_Medical_Wasteaste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.